Do you want to find 'write a balanced net ionic equation pbno32 h2so4'? You will find all the information on this section.
Table of contents
- Write a balanced net ionic equation pbno32 h2so4 in 2021
- Pb(no3)2 + h2so4 reaction type
- Pb(no3)2 + h2so4 precipitate
- Pb(no3)2 h2so4 pbso4 hno3 type of reaction
- H2so4 + pb(no3)2
- Net ionic equation calculator
- Lead 2 nitrate and sulfuric acid balanced equation
- Pb(no3)2 + h2so4 balanced equation
Write a balanced net ionic equation pbno32 h2so4 in 2021
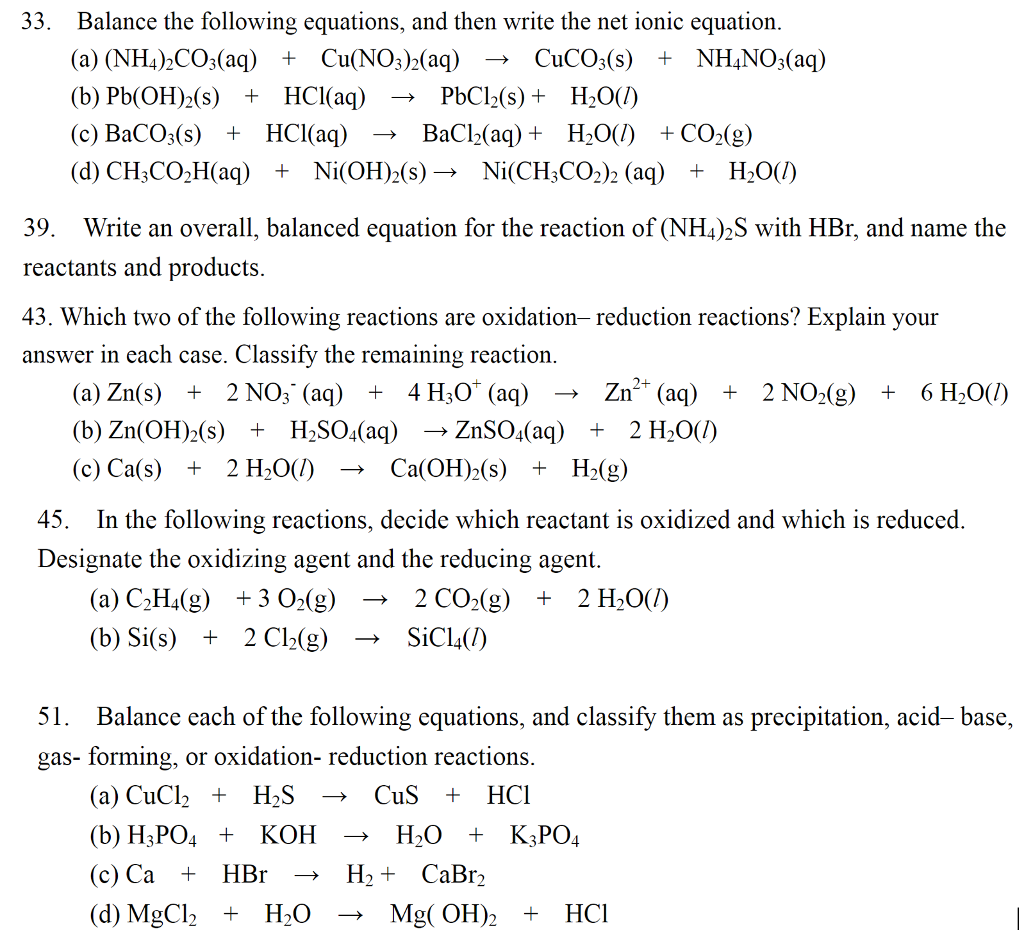 This picture illustrates write a balanced net ionic equation pbno32 h2so4.
This picture illustrates write a balanced net ionic equation pbno32 h2so4.
Pb(no3)2 + h2so4 reaction type
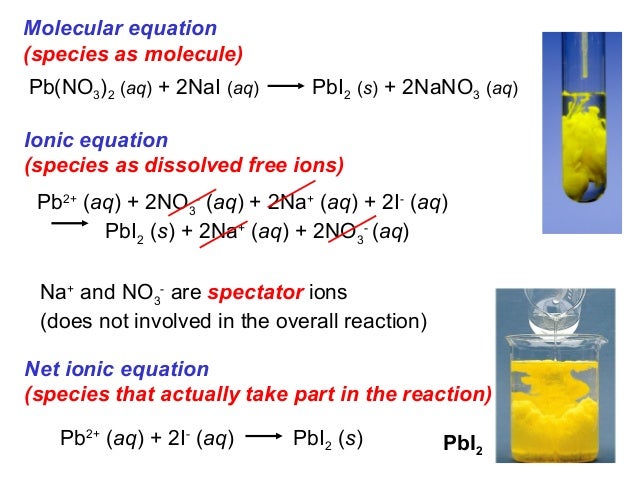 This picture representes Pb(no3)2 + h2so4 reaction type.
This picture representes Pb(no3)2 + h2so4 reaction type.
Pb(no3)2 + h2so4 precipitate
 This image demonstrates Pb(no3)2 + h2so4 precipitate.
This image demonstrates Pb(no3)2 + h2so4 precipitate.
Pb(no3)2 h2so4 pbso4 hno3 type of reaction
 This picture representes Pb(no3)2 h2so4 pbso4 hno3 type of reaction.
This picture representes Pb(no3)2 h2so4 pbso4 hno3 type of reaction.
H2so4 + pb(no3)2
 This image demonstrates H2so4 + pb(no3)2.
This image demonstrates H2so4 + pb(no3)2.
Net ionic equation calculator
 This picture demonstrates Net ionic equation calculator.
This picture demonstrates Net ionic equation calculator.
Lead 2 nitrate and sulfuric acid balanced equation
 This image demonstrates Lead 2 nitrate and sulfuric acid balanced equation.
This image demonstrates Lead 2 nitrate and sulfuric acid balanced equation.
Pb(no3)2 + h2so4 balanced equation
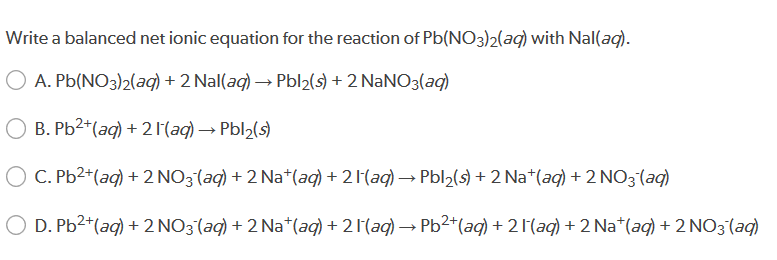 This image demonstrates Pb(no3)2 + h2so4 balanced equation.
This image demonstrates Pb(no3)2 + h2so4 balanced equation.
How to write the net ionic equation for H2SO4 + NaOH?
There are three main steps for writing the net ionic equation for H2SO4 + NaOH = Na2SO4 + H2O (Sulfuric acid + Sodium hydroxide). First, we balance the molecula...
How to write the net ionic equation for Pb ( NO3 ) 2 + NaCl?
There are three main steps for writing the net ionic equation for Pb (NO3)2 + NaCl = PbCl2 + NaNO3 (Lead (II) nitrate + Sodium chloride). First, we balance the molecular equation. Second, we write the states and break the soluble ionic compounds into their ions (these are the strong electrolytes with an (aq) after them).
What is the correct net ionic equation Ba ( OH ) 2?
Net Ionic Equation Ba (OH)2 (aq)+H2SO4 (aq)→ I have tried to answer this and it keeps saying its wrong can you please help. What is the correct net ionic equation, including all coefficients, charges, and phases, for the following set of reactants?
Last Update: Oct 2021